Pre-commercial Procedure
OncNGS consortium will challenge the market launching a pre-commercial procurement procedure, a competitive process enabling the buyers to compare the developments carried out by the contracted suppliers.

OncNGS consortium will challenge the market launching a pre-commercial procurement procedure, a competitive process enabling the buyers to compare the developments carried out by the contracted suppliers.
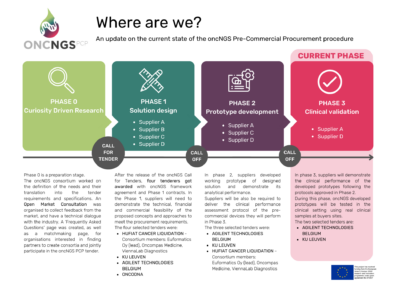
Phase 0 is a preparation stage. Before the opening of the Call for tender, we defined the needs by collecting feedback of the buyer’s group during the OMC (Open Market Consultation) and translate them into requirements and specifications to direct analysis and technical dialogue with the industry. The OMC is now closed. A ‘Frequently Asked Questions’ page has been created to gather and respond all the questions we received during the OMC. A matchmaking page is also available for organisations interested in finding partners to create consortia and jointly participate in the oncNGS PCP tender.
The oncONGS call for tenders is closed! Learn more. A Suppliers Information Day has been organised on the 29 June 2022 (the recording of the webinar is available here).
Following the preparation phase, including the Open Market Consultation events, and the release of the oncNGS Call for Tenders, four tenderers got awarded with oncNGS framework agreement and Phase 1 contracts. In the Phase 1, suppliers will need to demonstrate the technical, financial and commercial feasibility of the proposed concepts and approaches to meet the procurement requirements.
Duration: 4 months
Development of the well outlined and functioning prototypes.
In phase 2 suppliers will develop working prototype of designed solution and demonstrate its analytical performance.
Suppliers will be also be required to deliver the clinical performance assessment protocol of the pre-commercial devices they will perform in Phase 3.
Overall duration : 15 months – Execution : 12 months
the functional and technical properties of the selected solutions will be evaluated in an operational environment.
Minimum of 2 pre-commercial devices will be deployed.
Overall duration : 18 months – Execution : 15 months
The oncNGS consortium groups several buyers in 5 EU countries to challenge the market to develop novel affordable solutions to provide the most advanced NGS tests for cancer patients. We wish to co-develop an EU innovative tender for liquid biopsy diagnostics based on complex NGS-driven DNA profiling within a pre-commercial procurement Horizon-2020 financed project.

Improving the Cancer Care with Innovative ctDNA Analysis The[…]
The Pre-Commercial Procurement (PCP) project OncNGS has entered its[…]

On the 14, 20 and 26 March 2024, the[…]