OncNGS enters Phase 3 of the PCP with two innovative solutions for cancer NGS testing
The Pre-Commercial Procurement (PCP) project OncNGS has entered its final phase with two cutting-edge solutions for Next-Generation Sequencing (NGS) cancer tests.
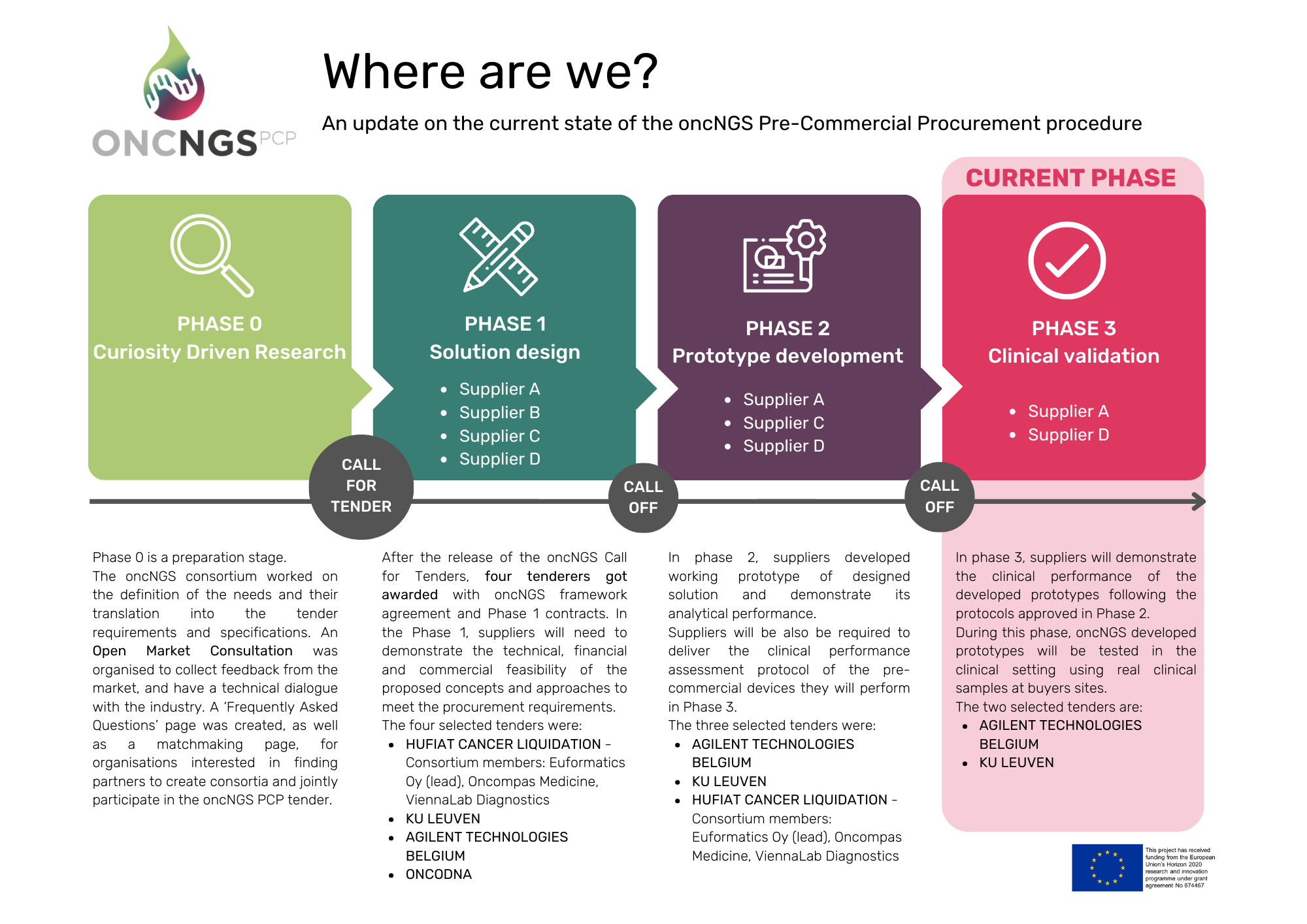
The Call-off for Phase 3 was launched on October 28, 2024, inviting the three successful suppliers from Phase 2 to submit an offer. After a rigorous evaluation process, the OncNGS procurers selected two suppliers to advance to enter Phase 3, where the clinical performance of their prototypes will be assessed.
Contracts for Phase 3 were awarded to:
Testing prototypes in real-world setting
Phase 3 officially started at the beginning of January 2025 and will run over the next 15 months. During this period, the selected suppliers will demonstrate the clinical performance of their prototypes in real-world clinical settings. Testing will be conducted both at the suppliers’ facilities and across seven pilot sites within the OncNGS buyers’ group:
- Institute Curie (IC)
- Institut Català d’Oncologia (ICO)
- ACC – Istituto Europeo di Oncologia (IEO)
- ACC – Istituto Nazionale Tumori Regina Elena (IRE)
- Institut Jules Bordet (IJB)
- Ludwig-Maximilians-Universität München (LMU)
- Hospices Civils de Lyon (HCL)
Advancing from prototypes to clinical validation
The Phase 3 call-off serves as a critical selection process within the PCP framework, ensuring the most promising solutions progress to full-scale clinical testing. Following a comprehensive evaluation, AGILENT and KU Leuven were awarded Phase 3 contracts to demonstrate the clinical, technical, financial, and commercial feasibility of their solutions. This final phase will determine the readiness of the developed NGS solutions for practical application in oncology, paving the way for advanced diagnostic tools in cancer care.
Articles récents
- Special Communication on oncNGS PCP published in ESMO Open
- OncNGS enters Phase 3 of the PCP with two innovative solutions for cancer NGS testing
- oncNGS consortium conducted site visits to phase 2 contractors
- OncNGS has entered in prototyping phase (Phase 2) with the selection of three innovative approaches for future Next-Generation Sequencing (NGS) tests for cancer
- Update on the current stage of the oncNGS Pre-Commercial Procurement procedure