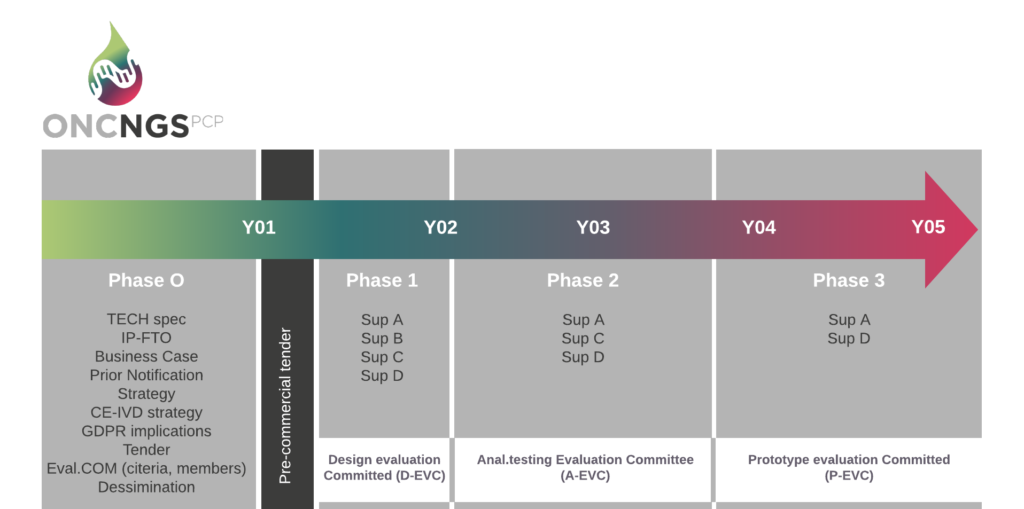
During this phase of the project, the oncNGS consortium will work on the definitions of the needs and their translation into the tender requirements and specifications.
In the Phase 0 will be organized an Open Market Consultation (OMC) in which the scope of the oncNGS pre-commercial procurement will be refined through a dialogue with potential suppliers and other stakeholders. The OMC will help us to collect insight into the market, including the state of the art and current developments. The outcome of this phase will be the preparation of a Call for Tender (CfT). The CfT will remain open for submission of tenders for at least 60 days. OncNGS CfT shall be open to any interested suppliers, with no need for a special invitation or specific pre-qualification requirements.
The pre-commercial procurement procedure consists of three clearly defined phases: Solution design, Prototype development and Clinical validation of a limited scale pre-commercial devices (see graphic above).