The project
OncNGS consortium wishes to develop novel affordable solutions to provide the best NGS tests, for all solid tumours / lymphomas patients, forever. Thanks to the provided solutions, oncNGS consortium wish to address their common identified unmet medical needs:
- Establishment of valuable common tumour profiling strategy allowing to provide equal access to innovative medicines to all;
- Enable to perform outcome research analysis after treatments with targeted therapies as diverse testing leads to lowering the pooling capacity of obtained results, needed to obtain large enough sample numbers to perform statistics analyses;
- Application of such essential testing to all patients, breaking down current unacceptable inequities due to the high costs of current diagnostics tests.
The challenge will consist in providing:
- efficient molecular DNA/RNA profiling of tumour-derived material in liquid biopsies by means of
- pan-cancer tumour marker analysis kit including NGS analysis integrated with
- an ICT decision support system including analytical test interpretation and reporting.
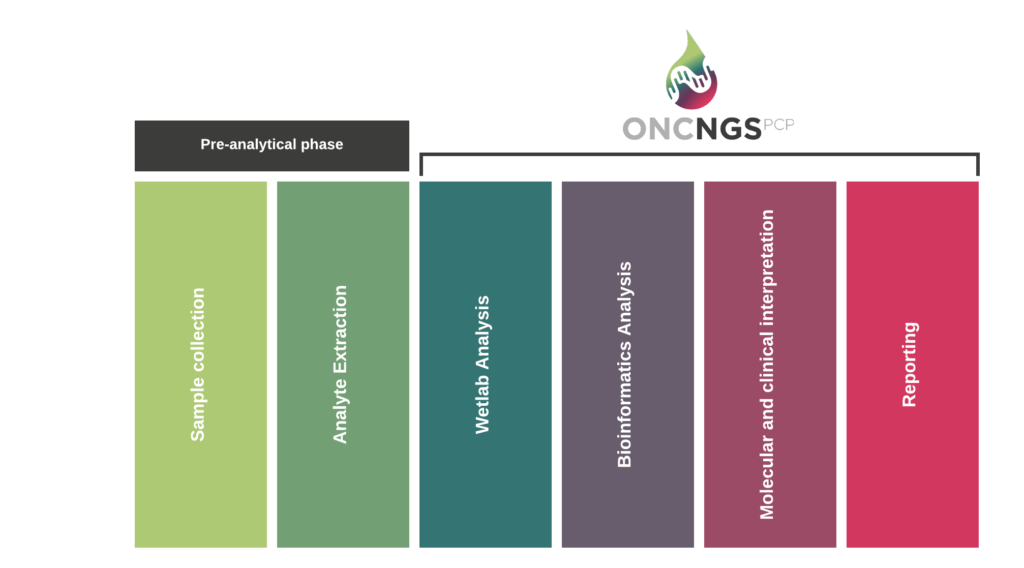
The figure above is picturing the overall process of DNA/RNA profiling of samples from cancer patients, from sample collection to reporting. The pre-analytical phase consisting in isolation of DNA/RNA analyte from samples (tissue of blood specimen) is out of scope of the oncNGS project. OncNGS starts with the wetlab steps for preparation of the NGS analysis. The generated sequences are then bioinformatically compiled and different types of variants are being identified. All relevant variants are then integrated into a report that will be used by the clinicians in their interpretation and diagnosis of the tumor.
The consortium
 OncNGS is a strong consortium composed of 8 buyers from 5 member states
OncNGS is a strong consortium composed of 8 buyers from 5 member states
- Sciensano (Belgium)
- Institut Jules Bordet (Belgium)
- Institut Curie (France)
- Hospices Civils de Lyon (France)
- Charite Universitaetsmedizin (Germany)
- Ludwig Maximilians Universitaet Muenchen (Germany)
- Alleanza Contro il Cancro (Italy)
- Institut Catala d’Oncologia (Spain)
Supported by 6 entities with wide experience in their fields :
- Agència de Qualitat i Avaluació Sanitàries de Catalunya (AQuAS), expert in pre-commercial procurement (Spain)
- Belgian Cancer Registry, expert in cancer control (Belgium)
- Institut National du Cancer, expert in cancer control (France)
- Instituto de Investigación Biomédica de Salamanca, expert in biomedical research in the field of haematologic malignancies (Spain)
- Valle de Hebrón Instituto de Oncología, expert in biomedical research in the field of haematologic malignancies (Spain)
- De Clercq & Partners, expert in intellectual property rights and freedom to operate analysis (Belgium)






