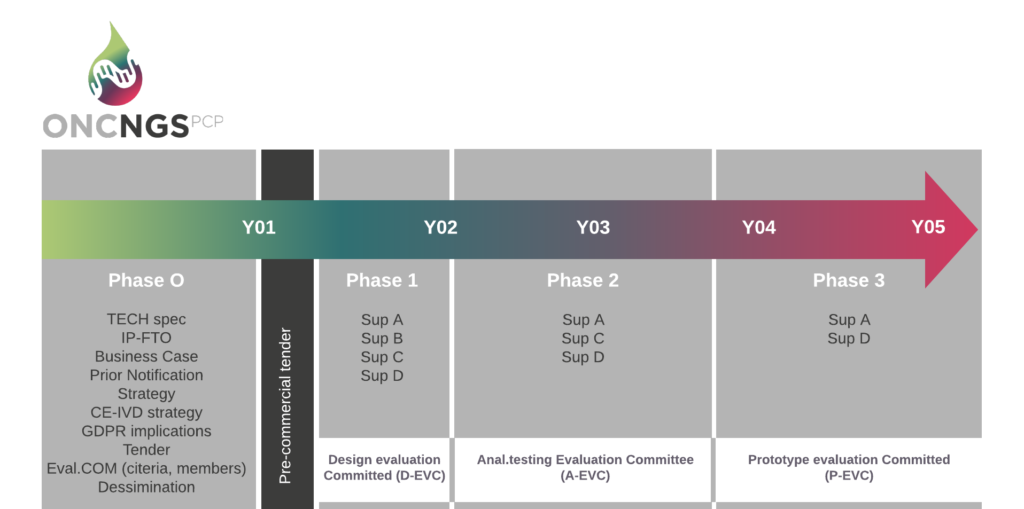
The pre-commercial procurement procedure consists of three clearly defined phases: Solution design, Prototype development and Clinical validation of a limited scale pre-commercial devices (see graphic below).
Phase 1 – Solution design: concepts elaboration into the competitive solutions. Minimum 4 designs will be selected.
Phase 2 – Prototype development: development of the well outlined and functioning prototypes. Minimum 3 designs will be selected.
Phase 3 – Clinical validation of a limited scale pre-commercial devices: the functional and technical properties of the selected solutions will be evaluated in an operational environment. Minimum of 2 pre-commercial devices will be deployed.